Adverse events should be reported. Reporting forms and information can be found at www.yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Nova Laboratories Ltd, Martin House, Gloucester Crescent, Wigston, Leicester, LE18 4YL Email: qa@novalabs.co.uk
Why a liquid suspension?
For young children, liquid formulations are the preferred choice for parents and healthcare professionals alike.(8) They provide a convenient method of administration for children as they are easy to swallow(9,10,11) and Jayempi’s sweet banana flavour reduces fussiness and improves treatment adherence. (1,3,5,12) What’s more, liquid formulations provide maximum dosing flexibility compared to solid, single-unit dosage forms over a wide age range.(3,4,13)
In a bioequivalence study, Jayempi demonstrated no clinically relevant difference in the blood concentration of the active metabolite mercaptopurine, compared to azathioprine tablet.(14) Therefore, no differences in the safety or efficacy are expected between the liquid and tablet formulation.(1,14)

Personalised dosing
Polymorphisms in the genes that encode the various enzymes involved in the metabolism of azathioprine may predict adverse reactions with azathioprine therapy.(1) Therefore, routine genotype/phenotype testing of thiopurine S-methyltransferase (TPMT) and nudix hydrolase15 (NUDT15) is recommended before initiating Jayempi to optimise treatment outcomes.(1,15,16,17,18)
Dose adjustments may be required following phenotype/genotype testing.(1,18,19) Making dose adjustments with solid oral formulations, for example, by splitting or crushing an unscored tablet, can result in dosing inaccuracies, as the active ingredient may not be uniformly distributed throughout the tablet.(3,4,20)
Efficacy and safety
Maintenance immunosuppression in regimens including azathioprine in low-risk renal transplant recipients is effective and well-tolerated.(7)
Studies show that azathioprine and mycophenolate are equally effective in preventing transplant rejection(6,22,23,24) and have a similar long-term risk/benefit profile when given in combination with oral steroids.(6,24)
In children and young people undergoing renal transplants, there are reports of increased rates of acute rejection in early steroid withdrawal with mycophenalate regimens when compared to azathioprine regimens, and more adverse events were reported with mycophenolate regimens compared with azathioprine regimens.(25)
The most important adverse reactions of azathioprine are bone marrow suppression, hepatotoxicity, nausea and vomiting.(26,27,28)
The effects of bone marrow suppression include leucopenia, thrombocytopenia and macrocytosis of erythrocytes.(29)
These adverse reactions are generally dose-dependent and resolve in seven to ten days with dose reduction.(27)
Mode of action
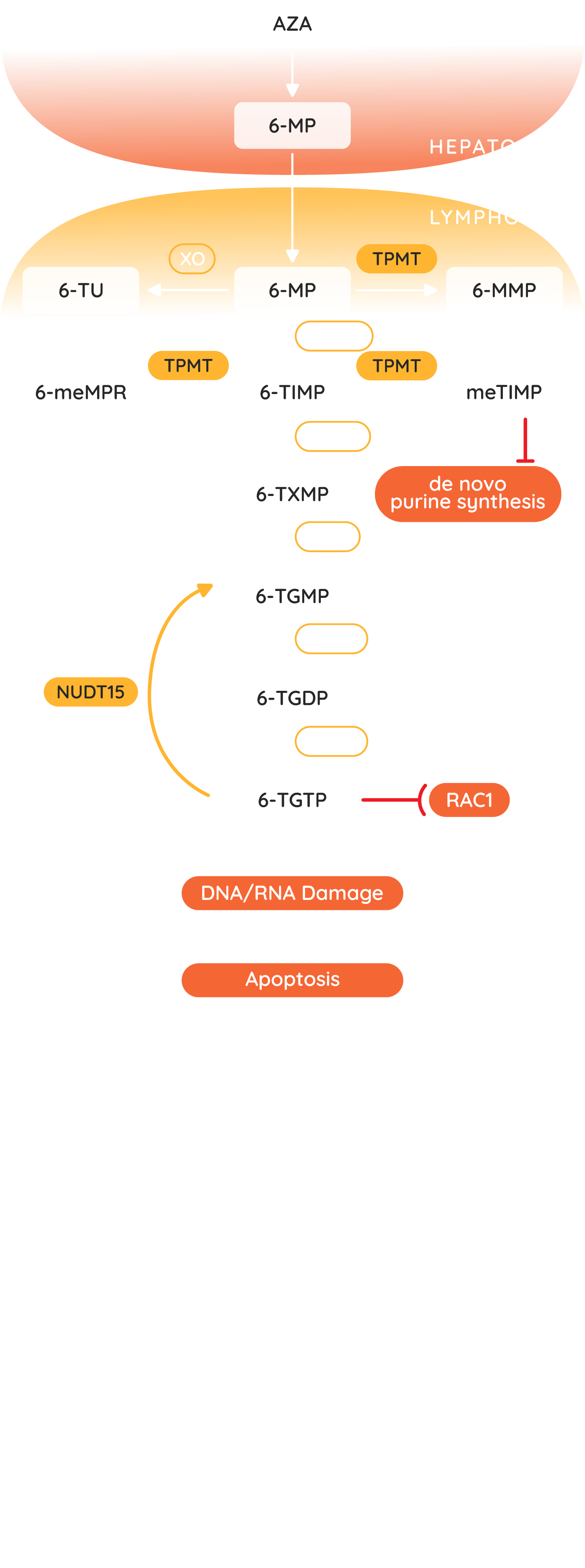
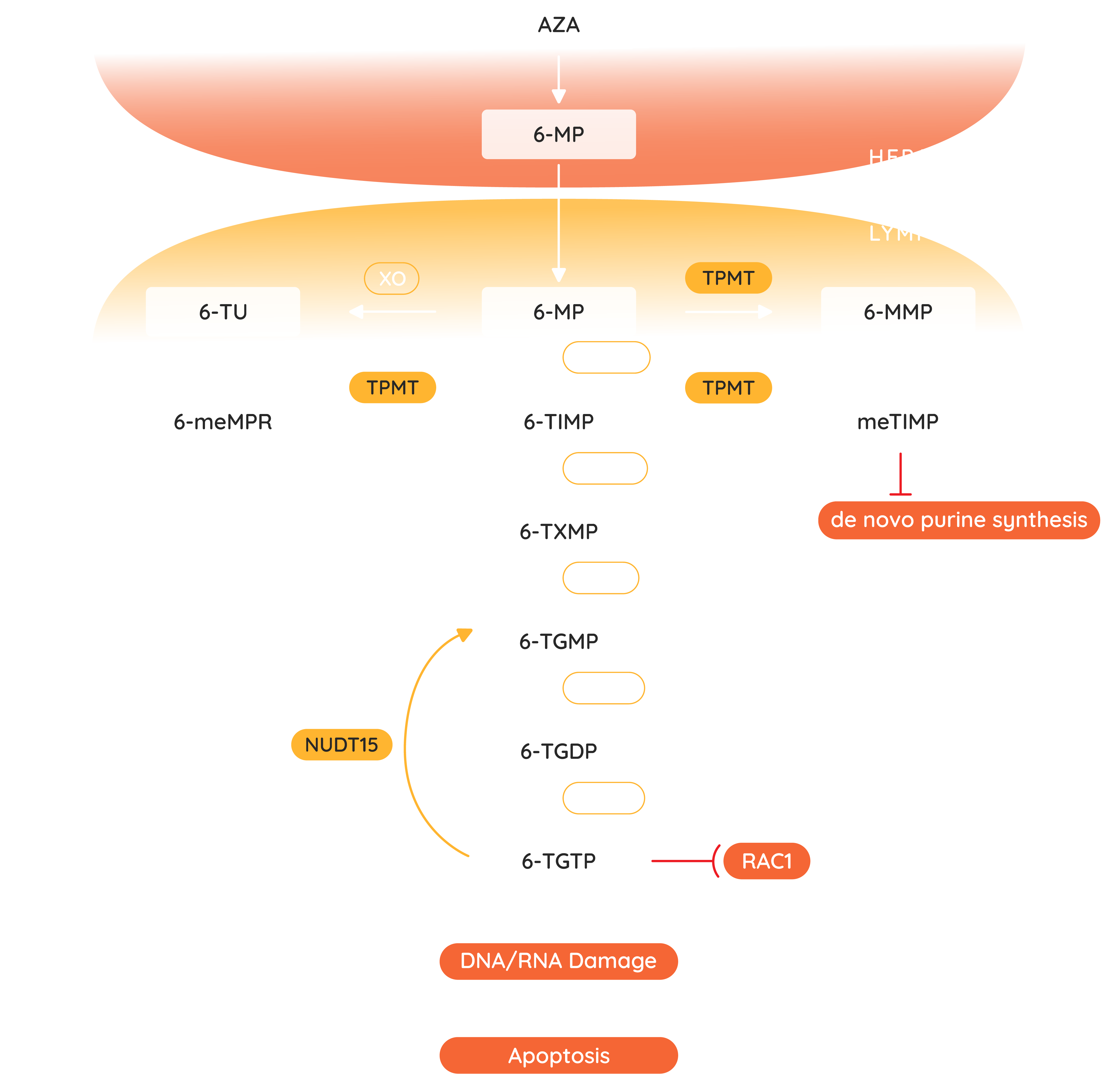
Jayempi (azathioprine) is an inactive pro-drug of 6-mercaptopurine (6-MP).(1) It has a multistep metabolic process into active and inactive metabolites. 6-thioguanine nucleotides are the active metabolites, which act as a purine antagonist and inhibit B and T lymphocyte activity via inhibition of DNA and RNA synthesis.(1,26,30,31) Azathioprine is a slow acting drug, and typically takes three months of therapy before a therapeutic effect is observed, although the toxic effects may occur any time during treatment.(18,32) It is therefore important to ensure the patient is educated on the importance of adherence to treatment.
View all Jayempi® SOT clinical information in one place
Downloads
These downloads (other than the HCP brochure) are only for use with patients prescribed Jayempi®.