Adverse events should be reported. Reporting forms and information can be found at www.yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Nova Laboratories Ltd, Martin House, Gloucester Crescent, Wigston, Leicester, LE18 4YL Email: qa@novalabs.co.uk

Efficacy and safety(1)
A clinical response to treatment with hydroxycarbamide may take 3-6 months and therefore, a 6-month trial on the maximum tolerated dose is required prior to considering discontinuation due to treatment failure (whether due to lack of adherence or failure to respond to therapy).
Haematological recovery usually occurs within two weeks of withdrawal of hydroxycarbamide. Gradual dose titration is recommended to avoid more severe bone marrow suppressions
Bone marrow suppression is the major toxic effect of hydroxycarbamide and is dose related
Hydroxycarbamide affects spermatogenesis and hence oligospermia and azoospermia are very commonly reported
Other commonly reported adverse effects also include nausea, constipation, headache, and dizziness
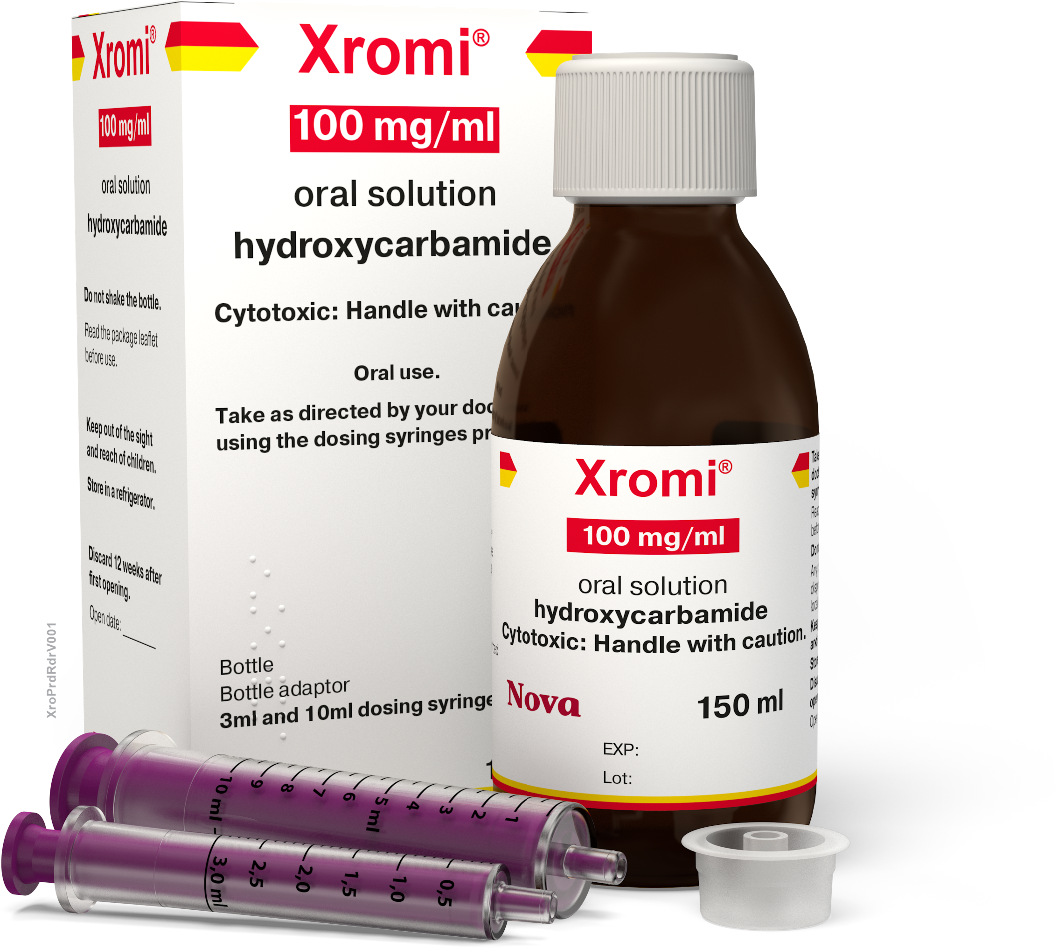
Hydroxycarbamide
Hydroxycarbamide has been shown to be an ideal drug with multiple mechanisms of action(5)
Hydroxycarbamide is a medicine that is taken orally. It causes changes in the blood to reduce the frequency of sickle cell crisis and the need for blood transfusions in patients with sickle cell disease. It is also sometimes known as hydroxyurea.(6)
There are several clinical papers that highlight the use of hydroxycarbamide for sickle cell anaemia, proving its efficacy over the past 25 years.(5)
*Images adapted from Guy’s & St Thomas‘ materials: Hydroxycarbamide for children with sickle cell disease(6)
Mode of action
The exact mechanism of action of hydroxycarbamide is unknown. However, blocking of the ribonucleotide reductase system which results in the inhibition of DNA synthesis appears to be the most important effect of hydroxycarbamide.(1) This effect causes cytotoxic suppression of erythroid progenitors and cell stress signalling which affects red blood cell production, encouraging the recruitment of erythroid progenitors with increased HbF levels.(5)
HbF interferes with the polymerisation of sickle haemoglobin which prevents the sickling of red blood cells.(1) Other known pharmacological effects of hydroxycarbamide that may benefit sickle cell disease patients include hydroxycarbamide-mediated alteration of the adhesion of red blood cells to the endothelium.(1)
Why is early initiation important?
It is recommended that all children over the age of 2 years with sickle cell disease are considered for treatment with hydroxycarbamide due to its impact of reducing the complications of the disease.(4)
Early initiation of hydroxycarbamide leads to sustained haematologic benefits and disease modification(4)
Stroke prevention
Stroke is the most devastating complication of sickle cell anaemia with a high recurrence rate, in both children and adults, if left untreated.(10)
In selected patients, transition to hydroxycarbamide therapy after 1 year of chronic blood transfusions is as effective as continuing chronic blood transfusions for the primary prevention of strokes in children with sickle cell disease.(10)
Stroke prevention
Transcranial Doppler (TCD) velocities ranges as follows:(4,10)
Normal <170 cm/sec (patients following a year of transfusions and with a normal TCD velocity range can be switched to hydroxycarbamide)
Conditional 170 – 199 cm/sec (hydroxycarbamide should be initiated and dose increased to maximum tolerated dose)
Abnormal ≥200 cm/sec (patients are at risk of stroke so require a number of transfusions)
Time in hospital and complete remission rates
The number of days in hospital is significantly lower when patients are on hydroxycarbamide therapy (range 0 to 19 days) compared to placebo (range 0 to 104 days)(3)
Risk reduction in childhood sickle cell mortality
Hydroxycarbamide leads to an 87% risk
reduction in childhood sickle cell disease mortality(11)
Why use a liquid formulation?
As children grow older, the way in which their bodies react to medicines and dosages varies.(14)
Child appropriate formulations must be able to adapt to these changes to provide children with safe and effective dosages.(14)
Oral solutions are considered the most appropriate formulation for young children and are the preferred choice for parents and healthcare professionals.(14)
Tablet splitting is associated with potential exposure of parents/carers to cytotoxic contamination.(15)
Liquid formulations provide maximal dosing flexibility making it possible to use over a wide age range(2)

View all Xromi® clinical information in one place
Downloads
These downloads (other than the HCP leaflet) are only for use with patients prescribed Xromi®.